Behavioral Science Essays
The
Attentional Blink
The
Glutamate Theory of Schizophrenia
Non-intelligent
Perception
Anxiety
and the GABA-A receptor subunit
On
The Theory-Ladenness of Observation
ECT in Depressed
Older Adults
Dennett Indented?
Anxiety, the GABAA receptor subunit isoforms, and benzodiazepines
Sten M. Andersen
Fear is a normal and adaptive response to threatening stimuli, but when fear is repeatedly experienced inappropriately, it might be characterised as an anxiety disorder (Bear, Connors, Paradiso, 2001); anxiety disorders are the most common of psychiatric disorders (Robbins & al., 1984). To treat anxiety, several substances have been used: e.g., alcohol, opiates, and barbiturates. Benzodiazepines (BZDs) were discovered in the early 1960s. They are anxiolytic, and are not as addictive as other substances that have been used in the treatment of anxiety (Leonard, 1992). BZDs are some of the most widely prescribed drugs (e.g., diazepam) (Rosenzweig, Leiman, Breedlove, 1999), to treat anxiety disorders, epilepsy, and to induce general anaesthesia (Williams & Akabas, 2000). BZDs work by increasing the channel-opening rates of the GABAA receptors (Study & Barker, 1981). As such, they have an effect on balance and coordination as controlled by the cerebellum, on the limbic areas and cerebral cortex (Leonard, 1992). In the present essay, GABA and the GABAA receptor will be introduced; the BZD receptor will be examined before the mechanisms of how BZDs bind to it are discussed. Then, the BZD receptor will be revisited and examined in greater detail. Several of the seven protein units that make up BZD-binding GABAA receptors will be discussed, but the focus will be on the α2 subunit. It will be argued that the α2 subunit of the GABAA receptor is important in mediating the anxiolytic effects of BZDs, and the distribution of α2 subunits in the brain will be touched upon. The methods of investigation are assessed before some side effects of BZD-treatment are considered. It will be concluded that even though some caution should be applied in interpreting the data, different subunit isoforms do seem to account for the different effects of BZDs. It will be suggested that these findings provide guidelines for the development of newer drugs with fewer side effects.
GABA and the GABAA receptor
γ-Aminobutyric acid (GABA) (C4H9NO2) is the most common inhibitory neurotransmitter in the brain; it is estimated to be present at about 40 % of the synapses (Rosenzweig, Leiman, & Breedlove, 1999). There are three large classes of GABA-receptors, A, B, and C (Rosenzweig et al., 1999). The GABAA receptor is the one that seems to be implicated in anxiety. The GABAA receptor is a pentameric (see Figure. 1), ligand-gated Cl-channel (MacDonald & Olsen, 1994). It has binding sites for GABA, BZDs, barbiturates, steroids, and picrotoxin (Sieghart, 1995; Smith & Olsen, 1995). The GABAA receptor belongs to a superfamily of ligand-gated ionotropic receptors (LGICs), including the nicotinic acetylcholine receptor, which the GABAA receptor seems to have evolved from (Schofield & al., 1987). In mammals, seven classes of subunits of this receptor have been identified, and most subunits have several isoforms: α1-6, β1-4, γ1-3, δ, ε, θ, and ρ1-2 (for loci of some subunits and their isoforms, see Table 2) (MacDonald & Olsen, 1994; Sieghart, 1995). Subunits from only one class (α) or two classes (α and β) can form functional GABA receptors under experimental conditions, but subunits from three classes (α, β, and γ) are needed for full receptor function (Miller, 1990); these three subunits also compose most of the GABAA receptors in the mammalian brain (Weinberger, 2001). With the use of these three, the most common isoform in the mammalian brain is (α1)2 (β2)2 (γ1) (Carlier, 2001).
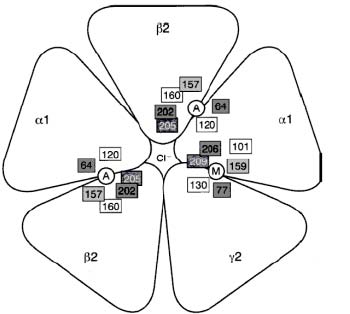
Figure 1. Proposed GABAA Receptor structure. Key: A: Agonist binding site. M: benzodiazepine binding site. Source: Carlier, 2001.
Table 1 Loci of subunit isoforms The benzodiazepine receptor BZDs do not bind to the GABA receptor directly, but to a BZD receptor site on the GABAA receptor complex (GARC). When doing so, BZDs allosterically modulate the GARC, augmenting the effects of GABA (Sandford, Argyropoulos, & Nutt, 2000); that is, it increases the effectiveness of GABA for opening the ion channel by changing the GARC’s shape (Carlier, 2001). The BZD receptor is the only known receptor for which there are not only agonists (e.g., diazepam, midazolam, flurazepam), partial agonists (e.g., RO 16-6028), and antagonists (e.g., flumazenil or RO 15-1788), but also full and partial inverse agonists (e.g., respectively, RO 19-4603, and RO 15-4513) (Jackson & Nutt, 1992; Leonard, 1992). The inverse agonists are anxiogenic. Since BZD allosterically modifies the GARC, the terms ‘agonist’ and ‘inverse agonist’ have been criticised. Instead, the terms ‘positive allosteric modulator’ and ‘negative allosteric modulator’ have been proposed (Puia, Vicini, Seeburg, & Costa, 1991, as cited in Chebib & Johnston, 2000). No matter what terms are used, this complexity has generated a lot of interest in the workings of BZDs and their receptor.
| Isoform | Loci | Source |
| α1 | cortical areas and thalamus | Rudolph et al., 1999 |
| olfactory bulb, neocortex, hippocampal | ||
| formation, cerebellum | Tecott, L. H., 2000 |
|
| α2 | limbic system | Löw et al., 2000 |
| central nucleus of amygdala | Rudolph et al., 2001 |
|
| “axon initial segment of principal |
α3 |
reticular activating system
|
Rudolph et al., 2001, p. 193
|
|---|---|---|
α5 |
limbic system |
Löw et al., 2000 |
α6 |
cerebellar granule cell |
Rudolph et al., 1999 |
β3 |
most brain areas |
Rudolph et al., 2001 |
δ |
cerebellum & thalamus |
Rudolph et al., 2001 |
The binding of benzodiazepines
As mentioned above, the GABAA receptor is a ligand gated ion channel (LGIC), and as other LGICs, it is composed of five peptide units; it is pentameric (Carlier, 2001). Each unit has an extracellular NH2 ending (the N-terminal domain) of about 200 amino acids (Carlier 2001; MacDonald & Olsen, 1994), four transmembrane domains M1-M4, a large extracellular loop between M3 and M4, and an extracellular COOH ending (the C-terminus) (Carlier, 2001), see Figure. 2. The N-terminal extracellular domain of some α units is necessary to bind BZDs (Norman et. al, 1990). Of the α subunits, only 1, 2, 3, and 5 bind to BZDs (Smith & Olsen, 1995). The γ2 subunit is also necessary for the GABAA receptor to be able to responds to low concentrations of BZDs (Rudolph, Crestani, & Möhler, 2001). Hence, we are left with a picture in which the α1, 2, 3, or 5 subunit, a γ2 subunit, and the N-terminal of one of the α subunits are necessary for the high affinity binding of BZDs that is normally observed in mammals. It has been hypothesised that BZDs bind at a binding site that lies at the interface between the γ2-subunit and an α1, 2, 3, or 5 subunit (Sigel & Buhr, 1997) (see Figure. 1).
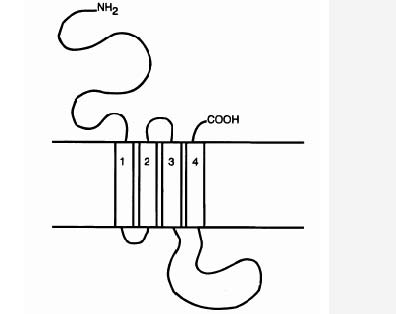
Figure 2. LGICS are composed of five peptide units. Each unit has an N-terminal domain, four transmembrane domains, M1-M4, a large extracellular loop between M3 and M4, and a C-terminus. Source: Chebib & Johnston, 2000.
Variations in composition of the subunits and the effects for BZD binding; analysis by gene-knockout strategies
Knockout mutations may be useful for giving us clues about the molecular basis of anxiety. If a receptor subunit is removed, some information about that subunit could be inferred from observing the changes in the physiology and pharmacology of the mutants (Rudolph, Crestani, & Möhler, 2001). δ–knockout mice, for example, show no differences from wild-type mice in the responses to midazolam (a water-soluble imidazo benzodiazepine), which suggests that there are no BZD binding sites in receptors containing δ–subunits (Rudolph et al., 2001). β3-knockout mice have only half the normal density of GABAA receptors in the brain, and most die in the neonatal period. But in those who live, there is a decreased effect of midazolam in impairing the righting reflex (Rudolph et al., 2001), suggesting that the β3-subunit is involved in the binding of BZDs. Mice in which the α6 subunit is knocked out do worse than wild-type mice in an accelerating rotarod test when exposed to diazepam (Korpi et al., 1999), but the findings are confounded because it seems that α6 subunits are co-assembled with δ–subunits, and so the absence of α6-subunits causes several changes in the cerebellum, including upregulation of a K+ channel and downregulation of other GABAA receptor subunits (Nusser et al., 1999). There is evidence, however, that many α-subunits are involved in the actions of BZDs (see below). That the γ2-subunits also play a role in BZD binding may be inferred from the finding that in two-week-old homozygous γ2-knockout mice, diazepam does not induce sedation, nor does it impair the righting reflex (Rudolph et al., 2001). In heterozygous γ2-knockout mice, synaptic clustering of GABAA receptors is reduced by 15-30 %; these mice show high anxiety to both learned and natural aversive stimuli (Smith & Olsen, 1995). Rudolph et al. (2001) points out that a reduced number of GABAA receptors also have been shown in patients with panic-type anxiety.
Variations in composition of the subunits and the effects for BZD binding; analysis by gene-knock-in strategies
The information above on effects of subunit composition on BZD binding was obtained using knockout mice; but this methodology has its flaws (as discussed below, “Assessment of the evidence”). Another way of acquiring information is by knock-in-point mutations, in which a single amino acid codon (consisting of three DNA base pairs) is changed into a codon for a different amino acid in vivo (Rudolph, Crestani, & Möhler, 2001). The mutant mice used in knock-in experiments are often named with a single letter amino acid code, with residue first, then residue number of the mature protein, and lastly, the mutant residue (e.g., α1H101R) (Teissére & Czajkowski, 2001). By using knock-in strategies, six residues in the γ2 subunit have been shown to be involved in the binding of BZDs. These residues will not be discussed further in this essay, but are listed in Table 2. All sources are as cited in Teissére and Czajkowski (2001).
Table 2 γ2 subunit shown to be involved in the binding of BZDs
Residue Source
γ2F77 (Buhr et al., 1997; Sigel et al., 1998) γ2A79 and γ2T81 (Kucken et al., 2000)
| Anxiety and the benzodiazepines | 9 | |
|---|---|---|
| γ2M130 | (Buhr and Sigel, 1997; Wingrove et al., 1997) | |
| γ2M57 and γ2Y58 | (Buhr and Sigel, 1997; Kucken et al., 2000) | |
The knock-in technique was also used by Wieland, Lüddens, & Seeburg (1992) to show that the histidine residues of the α1, 2, 3, and 5 subunits of GABAA receptors are necessary for the receptors to be sensitive to BZDs. The α1, 2, 3, and 5 subunits all have a conserved histidine residue in the drug-binding domain (Möhler et al., 2000), and by converting the histidine residue to an arginine residue by point mutation, it is possible to create mouse lines (e.g., α 1H101R, α 2H101R, α 3H126R) that do not show BZD effects specific to the receptor subtype that contains the mutated α-subunit (Rudolph et al., 2001). McKernan et al. (2000) used these findings to show that the α1 subunit mediates sedation. They found that the modified mice (α1H101R) did not react to the sedative properties of diazepam. In the same manner, Löw et al. (2000) used these mouse lines to investigate which α-subunits, specifically, were involved in the attenuation of anxiety. To assess the effects of an anxiolytic, the light-dark box (LDB) may be used. In this test, wild type mice to which an anxiolytic drug has been administered will choose to stay longer or more often in the lit area, compared to mice to which no such drug has been administered (Rudolph et al., 2001). Using the LDB, Löw et al. (2000) found that mice with the α2H101R point mutation did not show the anxiolytic effect of diazepam (as shown in the LDB and the elevated plus maze), whereas the effect was present in mice with the α3H126R point mutation. They concluded that the α2 GABAA receptors probably mediate the anxiolytic effects of BZDs.
Some of the neuronal substrates of anxiety, and the distribution of α2 subunits in the brain α2 GABAA receptors are expressed in the limbic system (Löw et al., 2000), especially in the central nucleus of the amygdala (Rudolph, Crestani, & Möhler, 2001), areas that are believed to be involved the processing of emotions (Bears, Connors, & Paradiso, 2001). Infusion of BZDs directly into the amygdala blocks the conditioning of fear and the physiological response to danger (Weinberger, 2001). The finding that the α2 subunits may mediate the anxiolytic effects of BZDs fits nicely into this picture. Furthermore, α2 cells seem to exhibit a fair amount of control over the output activity of the cerebral cortex and the hippocampus (Rudolph et al., 2001). The septo-hippocampal system has been said to act as a comparator between expected and actual stimuli (Gray, 1982); when the actual stimuli does not match the expected stimuli, the system inhibits ongoing activity. Again, it is not surprising to find α2 cells in this area. As a sidenote, it may be mentioned that in hippocampal cells, the single channel conductance has been reported to be increased by BZDs. In other cells, it is their probability of opening that increases (Williams & Akabas, 2000).
Assessment of the evidence Knockout mutations may be useful for giving clues about the molecular basis of anxiety, but there are certain flaws to the methodology. As mentioned above, other changes might occur in the brain as a result of the mutation (e.g., the α6-knockout mice); in some cases, the mutations are by and large incompatible with life (e.g., β3- and γ2-knockout mice) (Rudolph, Crestani, & Möhler, 2001). Hence, this technique might be more useful for learning about receptor assembly and function than about one particular receptor (Rudolph et al., 2001). Also, in the mutated gene of GABAA receptor knock-ins, there is a neomycin resistance cassette (Wieland, Lüddens, & Seeburg, 1992), which can change expressions of neighbouring genes. Another problem inherent in all animal studies is in inferring from animals to humans. For example, the BZD receptor complex seems strongly plastic in animals, but few changes have been seen in post-mortem samples from human brains; it might be that the plasticity of this receptor complex is quite different in human and animals brains, even though the BZD receptors appear to be quite similar (Leonard, 1992). This said, it might be noted that Malizia et al. (1998) showed that there are changes in cerebral GABA-BZD receptors in patients with panic disorder.
Side effects of benzodiazepines Since the BZD that are used for their therapeutic effect bind to GABAA receptors with different protein subunit composition, effects other than those that are aimed for are nevertheless observed. Diazepam, for example, often used as an anxiolytic, is also anticonvulsant, myorelaxant, and amnesic; especially when administered to elderly people, sedation, decreased memory, motor incoordination and muscle weakness are observed (Leonard, 1992). Even though BZDs are not nearly as addictive as other drugs used in the treatment of anxiety, physical dependence may develop because of the adaptive changes that have taken place in the brain. Insomnia and anxiety following BZD discontinuation have often been observed; in some cases, even seizures and paranoid behaviour (Leonard, 1992). A list of withdrawal symptoms is given in Table 3.
Table 3 Withdrawal symptoms from BZDs. Source: Leonard (1992)
Symptom Type
Insomnia Psychological Anxiety Psychological Conclusion In the present essay, the benzodiazepines (BZDs) and their methods of action have been examined. BZDs allosterically modulate the GABAA receptor complex (GARC), augmenting the effects of GABA. The GARC has been described, and some of the seven protein subunits that may form a GARC have been examined. Using knockout and knock-in mice, the function of specific receptor-subunits has been illuminated. It has been related that a α1, 2, 3, or 5 subunit, a γ2 subunit, and the N-terminal extracellular domain of some α unit all are necessary to bind BZDs. In considering the subunits, the α1-subunit was briefly mentioned, it seems to be involved in the sedative effects of BZDs. But attention was directed towards the α2-subunit, which seems to be implicated in the alleviation of anxiety. The loci of GABAA receptors with α2-subunit was mentioned when the neuronal substrates of anxiety was looked at; α2-subunit are found in the limbic system and in the hippocampal area; both areas have earlier been linked to anxiety. The methods of using knockout and knock-in mice were assessed. It was concluded that even though both methods give valuable information, they have properties that should render us cautious when interpreting the data. Nevertheless, much has been discovered about the workings of BZDs on GABAA receptors and the roles of various subunit isoforms, and optimism about future progress does not seem unwarranted. It does seem like the subunit isoforms might account for the various effects displayed by BZDs. In the future, this provides directional guidelines for the development of more specific drugs, i.e., drugs with fewer side effects.
| Apprehension | Psychological |
| Irritability | Psychological |
| Dysphoria | Psychological |
| Paranoid behaviour | Psychological / bodily symptom |
| Seizures | Bodily symptom |
| Palpitations | Bodily symptom |
| Tremor | Bodily symptom |
| Vertigo | Bodily symptom |
| Sweating | Bodily symptom |
| Hypersensitivity to light, | |
| sound and pain | Perceptual disturbance |
| Depersonalisation | Perceptual disturbance |
References
| Bear, M. F., Connors, B. W., & Paradiso, M. A. (2001). Neuroscience: Exploring the brain. (2nd ed.). Maryland: Lippincott Williams & Williams. |
Browne, S. H., Kang, J., Akk, G., Chiang, L. W., Schulman, H., Huguenard, J. R., & Prince,
D. A. (2001). Kinetic and pharmacological properties of GABAA receptors in single thalamic neurons and GABAA subunit expression. Journal of Neurophysiology, 86, pp. 2312-22.
Carlier, P. R. (2001). The GABAA receptor. Retrieved May 13, 2002, from Virginia Tech, Department of Chemistry Web site: http://www.chem.vt.edu/carlier/IntroGABA.pdf
Chebib, M., & Johnston, G. A. R. (2000). GABA-activated ligand gated ion channels: Medicinal chemistry and molecular biology. Journal of Medical Chemistry, 43(8), pp. 1427-47.
Gray, J. A. (1982). The neuropsychology of anxiety: An enquiry into the functions of the septo-hippocampal system. Behavioural Brain Sciences, 5, pp. 469-84.
Jackson, H, C., & Nutt, D. J. (1992). Strain differences in sensitivity to the hypothermic effects of benzodiazepine receptor ligands in mice. Psychopharmacology, 109(3), pp. 365-8.
Korpi, E. R., Koikkaliainen P., Vekovischeva O. Y., Mäkelä R., Kleinz R., Uusi-Oukari M., & Wisden, W. (1999). Cerebellar granule-cell-specific GABAA receptors attenuate benzodiazepine-induced ataxia: Evidence from 6-deficient mice. European Journal of Neuroscience, 17, pp. 1685-1697.
| Leonard, B. E. (1992). Fundamentals of psychopharmacology. Queensland: Jacaranda Wiley Ltd. Löw, K, Crestani, F., Keist, R., Benke, D., Brünig, I., Benson, J. A., Fritschy, J-M., Rülike,. |
T., Bluethmann, H., Möhler, H., & Rudolph, U. (2000). Molecular and neuronal substrate for the selective attenuation of anxiety. Science, 290, pp. 131-4. MacDonald, R. L., & Olsen, R. W. (1994). GABAA receptor channels. Annual Review of Neuroscience, 17, pp. 569-602.
Malizia, A. L., Cunningham, V. J., Bell, C. J., Liddle, P. F., Jones, T., & Nutt, D. J. (1998). Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Archives of General Psychiatry, 55(8), pp. 715-20.
McKernan, R. M., Rosahl, T. W., Reynolds, D. S., Sur, C., Wafford, K. A., Atack, J. R., Farrar, S., Myers, J., Cook, G., Ferris, P., Garrett, L., Bristow, L., Marshall, G., Macaulay, A., Brown, N., & Howell, O., Moore, K. W., Carling, R. W., Street, L. J., Castro, J. L., Ragan, C. I., Dawson, G. R., & Whiting, P. J. (2000). Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nature neuroscience, 3(6), 587-92.
Miller, L. G. (1990). The molecular biology of anxiety: A status report. Journal of Clinical Psychopharmacology, 10(4), pp. 235-6.
| Möhler, H., et al. (2000). The benzodiazepine site of GABAA –receptors. In GABA in the Nervous System (D. L. Martin and R. W. Olsen, Eds.), pp. 97-112. Lippincott: Williams and Wilkins. |
| Norman, T., Burrows, G. D., & Olver, J. S. (1990). Theories of the aetiology of anxiety. In G. D. Burrows, M. Roth & R. Noyes Jr. (Eds.), The Neurobiology of Anxiety (2nd ed.) (pp. 1-53). Amsterdam: Elsevier. |
Nusser, Z., Ahmad, Z., Tretter, V., Fuchs, K., Wisden, W., Sieghart, W., & Somogyi, P. (1999). Alterations in the expression of GABAA receptor subunits in cerebellar granule cells after the disruption of the α6 subunit gene. European Journal of Neuroscience, 11, pp. 1685-97.
Robbins, L. N., Helzer, J. E., Weisman, M. M., Orvaschel, H., Gruenberg, E., Burke, J. D., & Reiger, D. A. (1984). Lifetime prevalence of specific psychiatric disorders in three sites. Archive of General Psychiatry 41(10), pp. 949-58.
Rudolph, U., Crestani, F., Benke, D., Brünig, I., Benson, J. A., Fritschy, J-M., Martin, J. R., Bluethmann, H., & Möhler, H. (1999). Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature, 401, pp. 796-800.
Rudolph, U., Crestani, F., & Möhler, H. (2001). GABAA receptor subtypes: Dissecting their pharmacological functions. Trends in Pharmacological Sciences 22(4), 188-94.
Sandford, J. J., Argyropoulos, S. V., & Nutt, D. J. (2000). The psychobiology of anxiolytic drugs. Part 1: basic neurobiology. Pharmacology & Therapeutics, 88, pp. 197-212.
Sieghart, W. (1995). Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacological Review, 47, pp. 181-234.
Sigel, E., & Buhr, A. (1997). The benzodiazepine binding site of GABAA receptors. Trends in Pharmacological Sciences, 18, pp. 425-9.
Smith, G. B., & Olsen, R. W. (1995). Functional domains of GABAA receptors. Trends in Pharmacological Sciences, 16, pp. 162-168.
Study, R. E., & Barker, J. L. (1981). Diazepam and (─)-pentobarbital: Fluctuation analysis reveals different mechanisms for potentiation of gamma-aminobutyric acid responses in cultured central neurons. Proceedings of the National Academy of Science USA, 78, pp. 7180-4.
Tecott, L. H. (2000). Designer genes and anti-anxiety drugs. Nature neuroscience, 3(6), pp. 529–530.
Teissére, J. A., & Czajkowski, C. (2001). A β-strand in the γ2 subunit lines the benzodiazepine binding site of the GABAA receptor: Structural rearrangements detected during channel gating. The Journal of Neuroscience, 21(14), pp. 4977-86.
Weinberger, D. R. (2001). Anxiety at the frontier of molecular medicine. New England Journal of Medicine, 344, pp. 1247-9.
Wieland, H.A., Lüddens, H., & Seeburg, P. H. (1992). A single histidine in GABAA receptors is essential for benzodiazepine binding. Journal of Biological Chemistry, 267, 1426-9.
Williams, D. B., & Akabas, M. H. (2000). Benzodiazepines induce a conformational change in the region of the gamma-aminobutyric acid type A receptor alpha(1)-subunit M3 membrane-spanning segment. Molecular Pharmacology, 58(5), pp. 1129-36.
